Abstract
Background: Autoimmune cytopenias (AIC) frequently occur in chronic lymphocytic leukemia (CLL) patients and lead to shortened survival. The most common AICs in patients with CLL are autoimmune hemolytic anemia (AIHA) and immune thrombocytopenia (ITP). Treatments for CLL-associated AIC traditionally include corticosteroids, IVIG, rituximab, chemotherapy, and immunosuppressants. However, outcomes with these various treatments are not well established, especially in the era of small molecule inhibitors. This single-institution retrospective cohort study describes the patient characteristics associated with AIC, the frequency of different AIC treatments, and outcomes related to those treatments in one center.
Methods: The study included adults with CLL and at least one episode of AIHA or ITP who were treated at the Ohio State University between 1/1/2012 and 12/31/2019. Demographics, CLL disease history and features, and AIC episodes, type, treatment, and outcomes were collected. Laboratory values were collected at onset of AIC treatment and 6 weeks and 6 months after treatment to assess response. A recurrent AIC episode was defined as onset of AIC requiring treatment after >1 month without treatment for a previous episode of AIC. The primary endpoint was response to first treatment for the first AIC episode at 6 weeks. For AIHA, a response to treatment was defined as >2g increase in hemoglobin. For ITP, a response was defined as platelet count ³30,000 and complete response (CR) was defined as platelet count ³100,000. Logistic regression was used to associate patient characteristics with response. Overall survival (OS) was calculated from date of first AIC, and estimated using the method of Kaplan-Meier.
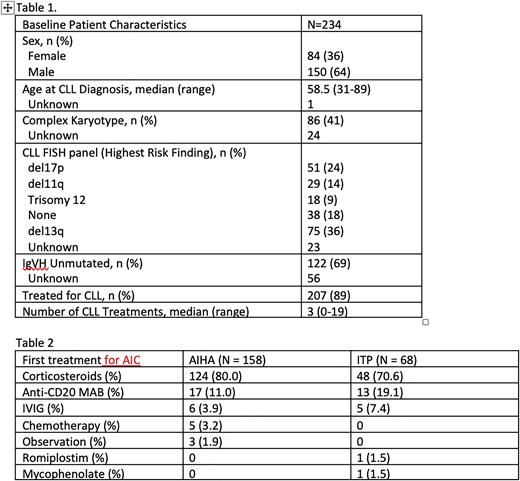
Results: The study population consisted of 234 patients. Median age at diagnosis of CLL was 58.5 years and median time from diagnosis of CLL to first AIC episode was 40 months (range -34 to 252). Baseline characteristics at CLL diagnosis are in Table 1. There were 4 patients with concomitant AIHA and ITP and 4 with pure red cell aplasia as a first AIC type, they were excluded from analysis of response to treatment
The first AIC was AIHA in 158 (68%) patients. The median number of treatments received for the first AIHA episode was 2 (range 0-7) with 3 patients (2%) not requiring treatment. The most common first treatments are described in Table 2. Of those with sufficient data to determine response, 57% (38 of 67) showed a response at 6 weeks and 84% (59 of 70) had a response at 6 months. The baseline median hemoglobin (g/dL) was 8.7 (range 3.0-12.1). This increased to at 6 weeks to 10.8 (7.7-14.3) and at 6 months to 12.5 (7.8-15.7). At least one recurrence occurred in 47 (30%) patients and there was no difference in first treatment type and the likelihood of having multiple episodes (p=0.32).
The first AIC was ITP in 68 (30%) patients. The median number of treatments for the first episode of ITP was 2 (range 1-8). The most common first treatments are described in Table 2. Of those with sufficient data to determine treatment response, 92% (24 of 26) had a response and 42% (11 of 26) had a CR at 6 weeks, and 96% (25 of 26) had a response and 77% (20 of 26) had a CR at 6 months. At least one recurrence occurred in 19 (28%) patients. Compared to those with at least one recurrence of AIC, patients with only one episode were more likely to have received corticosteroids (78% vs 53%) and less likely to have received anti-CD20 monoclonal antibodies as first treatment (10% vs 42%) (p=0.04).
None of the patient characteristics studied showed a significant impact on response to first treatment at 6 weeks for AIHA or ITP. There was no difference in likelihood of response based on what first treatment was used. The median OS from AIC diagnosis was 10.1 years (95% CI 8-11.9) in patients whose first AIC was AIHA compared to 17.1 years (95% CI 10.9-not reached) for ITP (p=0.02).
Conclusions: In this cohort study there was a response to treatment at 6 weeks in 57% of patients with AIHA and 92% with ITP. For both AIHA and ITP none of the initial treatment types or patient characteristics showed significant differences in likelihood of response. More patients with only 1 episode ITP received corticosteroids as an initial treatment compared to those with recurrences. Continued detailed analysis of treatment type and outcome is necessary to understand how to optimize the treatment of this complication of CLL.
Disclosures
Huang:AstraZeneca: Other: Statistical support. Grever:Serono: Consultancy; Ascerta: Consultancy; Hairy Cell Leukemia Foundation: Membership on an entity's Board of Directors or advisory committees, Research Funding; Axio: Consultancy; Pharmacyclics: Consultancy; Innate Pharma: Consultancy; AstraZeneca: Consultancy. Hoffman:Pharmacyclics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Beigene: Consultancy, Membership on an entity's Board of Directors or advisory committees. Kittai:Abbvie: Consultancy; Astrazeneca: Consultancy, Research Funding; Beigene: Consultancy; Janssen: Consultancy. Woyach:Karyopharm Therapeutics: Research Funding; Schrodinger: Research Funding; Newave: Consultancy; Genentech: Consultancy; Pharmacyclics: Consultancy; ArQule: Consultancy; MorphoSys: Consultancy, Research Funding; Loxo@Lilly: Research Funding; AbbVie: Consultancy, Research Funding; Janssen: Consultancy; BeiGene: Consultancy; AstraZeneca: Consultancy. Rogers:Innate Pharma: Consultancy; Pharmacyclics: Consultancy; Beigene: Consultancy; AstraZeneca: Consultancy, Other: Travel Funding; Novartis: Research Funding; Janssen: Research Funding; AbbVie: Consultancy, Research Funding; Genentech: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal